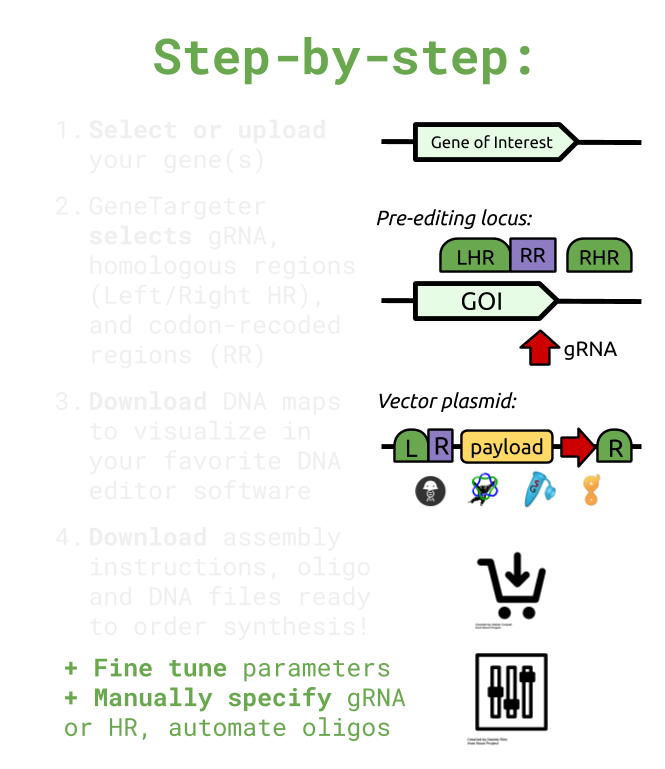
Creates custom gene-editing constructs designed to deliver DNA payload to specific genes in malaria parasites, including for knock-out or conditional knock-down in Plasmodium falciparum.
A detailed description of this tool is now published. Command line interface and code available through GitHub. Developed by the Niles Lab at MIT.